-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Mathu Raveendran T, A Abarnadevika* and G Ariharasivakumar
Corresponding Author: A Abarnadevika, Department of Pharmacology, KMCH College of Pharmacy, Coimbatore, Tamilnadu, India
Received: February 03, 2022 ; Revised: February 06, 2022 ; Accepted: February 08, 2022 ; Available Online: May 1, 2022
Citation: Mathu RT, A Abarnadevika and G Ariharasivakumar. (2022) A Study of Biguanides in the Care of Type II DIABETES Mellitus. J Pharm Sci Drug Discov, 1(1): 1-9.
Copyrights: ©2022 Mathu RT, A Abarnadevika and G Ariharasivakumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Type II diabetes mellitus (T-II-DM) is now a worldwide scourge which represents a huge test to medical services frameworks. This sickness is described by higher-than-normal blood sugar levels because of a combination of insulin resistance and insufficient of insulin discharges from the β-cells of pancreatic Islets of Langerhans. Metformin and phenformin, are the major biguanides, presented in 1957 as oral glucose-reducing agents for the treatment of NIDDM (non-insulin -dependent diabetes mellitus). Phenformin was removed in numerous countries as an aftereffect of a relationship with lactic acidosis, but metformin was not like a similar hazard on the off chance that suitably recommended. Biguanides are the first line antihyperglycemic agents, mainly metformin that works chiefly by controlling hepatic glucose production and peripheral insulin affectability. Metformin is currently generally utilized as a monotherapy and in mix with a sulfonylurea metformin has been clinically applied for the greater part a century, despite the fact that the hidden pharmacological mechanisms stay tricky. This current review mainly centered on the history of biguanides, pharmacokinetics, pharmacodynamics, mechanism of action, combination therapy and adverse effects.
Keywords: Type II diabetes mellitus, Biguanides, Insulin release β-cells
INTRODUCTION
The persistent metabolic condition of diabetes is primarily distinguished by the loss of homeostasis of carbohydrates with protein and fat metabolism disruptions that result from outcome from either insulin discharge or insulin activity deserts or both the protein (hormone) is insulin, synthesized pancreatic beta cells respond to multiple stimuli, such as glucose, sulphonyl urea, arginine, but the key determinant is glucose [1].
It is recognized that the most common factors donating to the pathophysiology of T-II-DM, a continuum of disease arising from tissue insulin blocking and ultimately progressing to a disorder characterized by complete loss of secretary function of the pancreatic beta cells, are insulin secretion deficiency, resistance to insulin tissue acts, or a mix of the two [1].
At present available medications for type II diabetes are include: biguanides, sulfonylurea, thiazolidinediones or glitazones, glucagon-like peptide-1 (GLP-1) agonists, dipeptidyl peptidase four (DPP-4) inhibitors, alpha-glucosidase inhibitors, glinides or meglitinides, sodium-glucose cotransporter- 2 inhibitors and insulin [1].
Biguanides increases insulin sensitivity, decreases fasting plasma sugar and insulin concentrations; in the lack of insulin, it is not effective. The glucose-reducing effect is primarily due to reduced development of hepatic glucose and increased peripheral glucose absorption in NIDDM patients. Type of diabetes is type 1, depending on insulin. Pancreatic islet beta cells are destroyed, with most cases being autoimmune antibodies (type IA) that destroy beta cells in the blood, but some are also idiopathic (type IB). Type II is a type of diabetes that is not insulin dependent. GLP-1 is an essential insulin-inducing incretin from pancreatic beta cells. This will inhibit the release of glucagon. The GLP-1 receptors are activated where appetite is suppressed [2].
Type II diabetes mellitus requires multiple processes and is a chronic progressive condition. Metformin is a derivative of biguanide that decreases the production of hepatic glucose as well as intestinal glucose absorption and increases insulin sensitivity by rising the absorption and use of peripheral glucose [3].
Type II diabetes commonly occurs in adults, but children are often affected by obesity as well as any other physiological and pathological disorders causing it. Because of its effect on small blood vessels that supply the kidney, nerves and eyes, diabetes is responsible for many complications, thereby causing nephropathy, neuropathy and retinopathy. Larger blood vessels are also impaired, resulting in angina pectoris, myocardial infarction, acute ischemic attacks, stroke, and peripheral arterial diseases [4]. Diabetics have dramatically raised oxidative stress levels and this is a major contributor to the majority of neurological, cardiovascular, retinal, and renal diabetic complications [5].
Chemically, metformin is an oral active medication for type II diabetes mellitus, N-dimethylimidodicarbonimidic diamide, belonging to the biguanide class, which works essentially by stimulating the enzyme AMPK, which further controls the metabolism of carbohydrates by falling intestinal glucose uptake, hepatic glucose making and increasing in success [6]. And it is commonly used by nearly 120 million people worldwide for the treatment of type II diabetes. Biguanides is the active compound of the Galega officinalis. In the 1920, they were first accepted and used as therapeutic agents in the 1950. Metformin was approved as an antihyperglycemic agent in the United Kingdom in 1958, Canada in 1972 and the United States in 1995 and is suggested as a first-line treatment for type II diabetes by the European Association [6].
The key effect of Biguanides is to reduce the output of hepatic glucose as an antihyperglycemic agent. In addition, it rises the usage of insulin-mediated glucose in peripheral tissues (e.g., liver and muscle), reduces the absorption of glucose in the small intestine and decreases plasma-free fatty acid concentrations, thus reducing the availability of gluconeogenesis substrates. As a consequence, it lowers the blood sugar levels in type II diabetes and does not cause excessive hypoglycemia. Metformin has recently been found to be effectual in treating multiple cancers, especially cancers of the prostate, colon, and breast cancer. Its biological half-life (t1/2) is in the 0.9-2.6 h range. For effective treatment, regular use of large doses of metformin (500 mg two or three times a day, or 850 mg once or twice a day with or after meals) is also essential [6]. With its approximate absolute bioavailability of about 50 to 60 percent, it is absorbed steadily and incompletely from the gastrointestinal tract, liberally soluble in water [7].
Worldwide, T-II-DM predominance has achieved outbreak proportions. As diabetes induces the risk of cardiovascular diseases and early mortality, T-II-DM prevention and management has become a major public health issue around the world [8]. The risk of diabetes rises day by day and is primarily found in women rather than in men [9]. 150 million people have diabetes, according to epidemiological studies, and 300 million people worldwide are expected to be affected by 2025 [10].
As oral glucose-reducing agents for the treatment of non-insulin dependent mellitus diabetes (NIDDM), the two biguanides, metformin and phenformin, were introduced in 1957. With its association with lactic acidosis, phenformin has been discontinued in many countries, but metformin does not bear the same risk if properly administered. Buformin was introduced in 1958 only restricted use was given to Buformin, but phenformin was broadly introduced in the 1960 and early 1970 [11,12]. Contact with lactic acidosis has resulted in the removal of phenformin in some countries [13,14], but it is still used in some countries with caution, either as monotherapy or in combination with sulfonylurea. Special interest in this has been demonstrated by a fixed combo tablet containing phenformin and glyburide [15]. Metformin, however, took precedence among the biguanides.
BIGUANIDES
Metformin: Somewhere else, the science, pharmacokinetics, and toxicology of metformin have been fully assessed, yet a few highlights merit emphasis. Assimilation of metformin happens primarily from the small digestive system [11]. The assimilation T1/2 measured is 0.9-2.6 h, and the bioavailability is 50-60%. Metformin centralization in fringe plasma reaches utmost of ~2 mg/ ml (~10~5 M) ~2 h later oral dosing (500 mg or 1 g) of the exclusive Glucophage brand (22-24 High metformin groupings (10-100 times plasma fixations) gathers in the gastrointestinal plot dividers, and concentrations occur in the kidney, liver, and salivary organs more significantly than twice above plasma [16]. Metformin is steady and does not bind to proteins in plasma. In the pee, it is discharged simply unchanged. The end is quick (evaluated plasma T1/2 is 1.7-4.5 h), with 90% cleared in 12 h. More interesting than the glomerular filtration rate is the renal independence of metformin, which recommends that the proximal tangled tubules emit the drug [17].
MECHANISM OF ACTION
Biguanides (Metformin) reaches cells fundamentally through the family of solute transporters 22 part 1 (otherwise called natural cation carrier 1 (hOCT1)) and applies different insulin dependent and insulin-autonomous activities agreeing to the degree of medication introduction and the control of supplement digestion inside various tissues [18]. During therapy, the gut is subjected to high metformin (biguanides) convergence, which interferes with the mitochondrial respiratory chain at complex I and increases the use of glucose, anaerobic glycolysis, and the development of lactate; a portion of the lactate can be switched back to liver glucose. Lactate-glucose turnover induces dispersal of vitality, which can lead to the non-partisan weight (absence of weight put on or weight misfortune) seen in patients treated with metformin. Metformin (biguanides) improves insulin flagging in the liver, reduces the activity of glucagon and decreases gluconeogenesis and glycogenolysis [19,20]. Metformin (biguanides) can interfere with the glycerol-3-phosphate dehydrogenase mitochondrial redox transport catalyst, change the hepatocellular redox state and lead to decreases in the proportion of ATP: AMP, hepatic gluconeogenesis, lactate and glycerol transformation to glucose, and action of AMP-enacted protein kinase (AMPK) [21]. Similarly, the treatment of metformin (biguanides) brings about a turn towards the use of glucose as a cell wellspring of energy in the liver relative to unsaturated fats [21]. Metformin (biguanides) promotes insulin-mediated glucose uptake in the muscle by solvent transporter family 2, facilitated by glucose carrier part 4 (GLUT-4) (Figure 1) [19].
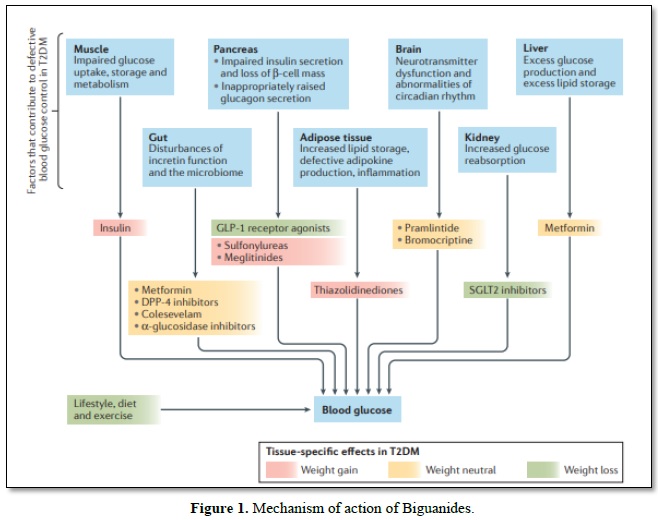
Glucose movement destinations cutting administrators down. Diverse hereditary and natural parts give an expansion in sort II diabetes mellitus (T-II-DM) through insulin resilience to pancreatic β-cell disappointment. Overweight and strength add to insulin opposition according to expanded fiery signals and upset lipid homeostasis, which consistently goes before the beginning of hyperglycemia for quite a long while and increments cardiovascular dangers At a phase where insulin outflow isn't satisfactory to control insulin impediment now, glucose extremism advances to T-II-DM, by and large joined with pancreatic ш cell break that incites glucagon release, diminished prandial emanation or development of incretin hormones, for example glucagon-like peptide 1 (GLP 1), changes in the intestinal microbiome, and disrupting impacts of neural exercise. Insulin, sulfonylureas and meglitinides are related with the danger of hypoglycemia. DPP 4, dipeptidyl peptidase 4; SGLT2, sodium/glucose co-carrier 2.
As delayed discharge plans for metformin have achieved comparable effectiveness at lower doses as compared to 'normal' plans, it appears that the intestine is a major site of metformin action at restorative doses. Metformin can increase the glucagon-like peptide-1 (GLP-1) circling levels from pre-treatment levels, even when an oral glucose load is not observed and in people with and without T-II-DM [21],by means of instruments that could integrate sodium-subordinated bile corrosive carrier restrictions, which increase the accessibility of ileal bile acids to initiate G-protein coupled bile corrosive receptor 1.When viewed with fake care, metformin decreases the movement of dipeptidyl peptidase 4 (DPP-4) [22]. Compared to pretreatment levels, metformin improves the discharge of GLP-1 to an oral glucose load through muscarinic (M3) and gastrin-delivery peptide receptor (GRP-R)-subordinate pathways.
In mice, metformin animates articulation of GLP-1 receptor (GLP-1r) on pancreatic β cells, interceded by peroxisome proliferator-actuated receptor (PPAR) α. The impact of metformin on GLP-1 may add to its weight-unbiased impact and to decrease in hepatic glucose yield by hindering glucagon secretion. Metformin likewise influences the circadian control of glucose digestion in liver and muscle [23-27].
Metformin mediated AMPK enactment results in the phosphorylation of casein kinase I, which causes the corruption of the circadian clock component mPer2, which subsequently improves the articulation of the circadian qualities of CLOCK and BMAL1, which also causes the advance of circadian musicality in treated rodents, contrasting and untreated controls. Consequences of the investigation, including mice, suggested that metformin induces improvement in the liver, but stage delay in muscle and the effects of metformin on circadian mood are closed in mice with Prkaa2 intake, the consistency coding AMPK subunit [28,29].
PHARMACOKINETICS
Biguanides (metformin) have 40-60 percent oral bioavailability and a half-life of plasma 4-9hr, and unchanged removal in urine mainly by tubular secretion instead of filtration by glomerulus [30].
PHARMACODYNAMICS
Biguanides (metformin) is widely used as a first-line pharmacotherapy in patients with T-II-DM, because of its efficacy, long-term safety record, low risk of hypoglycemia, weight neutrality and favorable impact on vascular disease [31]. Biguanides (metformin) therapy usually results in a reduction in fasting plasma glucose (FPG) by 2-4 mmol/l and HbA1c by 1-2 percent, largely independent of age, weight and T-II-DM length, as long as some residual β-cell function remains [32]. In the 10-year follow-up results from the UK Prospective Diabetes Trial (UKPDS), patients receiving biguanides (metformin) had substantial risk reductions of 21 percent (P=0.01) for any diabetes-related endpoint, 30 percent (P=0.01) for diabetes-related deaths, and 33 percent (P=0.005) for myocardial infarction relative to overweight patients in the traditional therapy community [32,33]. Biguanides (metformin) may also be associated with a reduction in the risk of cancer in patients with T-II-DM, especially prostate, pancreatic and breast cancer [20]. The progressive design of T-II-DM may involve the addition of other glucose-lowering treatments (including insulin) to biguanides (metformin). As a result, several fixed-dose combinations of drugs like biguanides (metformin) are available [34-36].
EFFECTS OF HEPATIC GLUCOSE OUTPUT
Exorbitant HGP (hepatic glucose production), a Major factor in type II diabetes mellitus pathogenesis. Treatment of biguanides with measurements of 1000 to 2550 mg / day for as long as a total of 3 months lowers basal HGP (normally 10% to 30%). Biguanides -initiated concealment of hepatic gluconeogenesis, interspersed mainly by decreased free unsaturated fat (FFA) and lipid oxidation, has been suggested as a key driver for the lowering of fasting hyperglycemia [37-39].
IMPACT ON THE BINDING OF INSULIN
Insulin official to cell-layer receptors is decreased in type II diabetes mellitus. Biguanides treatment has been appeared to maximize or modify the thickness and to limit the partiality of insulin receptors on erythrocytes and monocytes acquired by normoglycemic and diabetic people. Moreover, the degree of insulin receptor restriction does not give the impression that it is essentially associated with metabolic and clinical reactions to metformin therapy, indicating that these impacts are interceded at intracellular level. The implications for the restriction are high-limit, low-like receptors whose task in glucose digestion is unclear. As a result, the effects of biguanides on these receptors are most likely not to contribute significantly to the antidiabetic activity of biguanides [40].
EFFECTS ON THE PRODUCTION OF INSULIN
As stated before, as opposed to the sulfonylureas, biguanides doesn't invigorate production of insulin. Similarly, studies of hypertensive non-diabetic individuals and type II diabetic patients by and large reveal that the plasma levels of insulin and C-peptide are unchanged during biguanides, therapy. Notwithstanding, a few investigations have detailed noteworthy decreases in both plasma insulin and the insulin antecedent proinsulin diabetic patients with slim and overweight type II [41-46].
When in doubt, these are conceivably helper to glucose, which initiates the impacts of biguanides, not the prompt activity of the medication. Biguanides started decreases in fasting plasma insulin levels in both fit sort II diabetic patients [47] and overweight sort II diabetic patients going through attending insulin therapy were credited distinctively to diminish in insulin obstacle and exogenous insulin usage. Notwithstanding, the biguanides related drop in plasma levels of proinsulin in diabetic patients of type II was not viable with diminishes in body weight, fasting blood glucose levels or insulin resistance [48].
Continuous BIGPRO (Biguanides and Preventing the Risk of Obesity) preliminary investigates the impact of biguanides on atherogenic and diabetic variants of the android fat circulation standard primer discoveries show that biguanides can fundamentally decrease (by ú50%) fasting insulinemia in nondiabetic people [49].
HYPOGLYCEMIC MEDICATIONS COMBINATIONS
The different mechanisms of action of the various classes of hypoglycemic drugs makes combined therapy feasible: the sulfonylureas and meglitinides stimulate insulin production by different mechanisms, the biguanides reduce glucose production by the liver and excretion from the liver, acarbose reduces the absorption of glucose from the intestine, and the thiazolidinediones reduce insulin resistance in fat.
There is no need to wait until one drug has reached its maximum dose before starting another however, when the development of endogenous insulin is limited, sulfonylureas and meglitinides should no longer be used. Insulin combinations of sulfonylureas or meglitinides can only be used when the patient shifts to insulin, even when long-acting insulin is given at night to rest the islets and facilitate daytime insulin secretion [50].
COMBINATION TREATMENT OF BIGUANIDES AND SULFONYLUREAS
There has been thorough analysis of the synthesis of biguanides with various sulfonylurea derivatives. For patients with type II diabetes who were poorly regulated when metformin (biguanides) or pioglitazone was added to sulfonylureas, those with reduced pancreatic beta-cell function responded better to metformin, while those with higher insulin tolerance reacted better to pioglitazone [51]. The mix of metformin (biguanides) + glibenclamide (250 + 1.25 mg) tablets and metformin (biguanides) 500 mg / day or glibenclamide monotherapy was compared to 2.5 mg / day for approximately four months in 486 patients [52].
The complete day by day portions were balanced relying upon fasting plasma glucose. The last mean dose with joint therapy was lower than with monotherapy. Gastrointestinal side effects, for example, looseness of the bowels, sickness, heaving, and stomach torment, were essentially more continuous or metformin monotherapy as either consolidated therapy or glibenclamide monotherapy.
Hypoglycemia has usually been visited in individuals who have undergone combination therapy and least metformin monotherapy, but finger-stick glucose concentrations below 2.8 mmol / l were rare with metformin monotherapy and equally typical with glibenclamide monotherapy and combination therapy [53].
In the United Kingdom Prospective Diabetes Research, a subgroup of patients undergoing sulfonylurea therapy with metformin seems to have had an overabundance of mortality. Information from 263 general practices in the United Kingdom was broken down; at first, 8488 patients received sulfonylurea, including metformin in 1868 [54]. The unrefined death rates for 1,000-man years were 59 and 40 per man. Metformin was first used in 3099 patients and 867sulfonylurea was used. Gross death rates per 1,000-man years were 25 and 20 per man. These findings show that there is no increased risk of death with a combination of sulfonylurea and metformin.
COMBINATION TREATMENT WITH BIGUANIDES AND GLITAZONES
The combination of metformin (biguanides) + thiazolidinediones (glitazones) is contraindicated or not recommended in patients undergoing cardiovascular disappointment therapy. In the review investigation of 12,505 and 13,158 patients with cardiovascular disappointment and diabetes in two separate years (1998/9 and 2000/1), 7.1 per cent (later 11 per cent) of patients had, biguanides solutions, 7.2 per cent (16 per cent) thiazolidinediones and 14 per cent (24 per cent) cardiac supplements. This is indicated for the consumption of hypoglycemic drugs in certain patients with cardiovascular breakdown despite contraindications [55].
COMBINATION TREATMENT WITH BIGUANIDES AND INSULIN
While, biguanides expansion (2.5 g / day for 2 month) reduced daily insulin pre-requisite by 16 per cent in the set of insulin treated NIDDM patients, little thought has been given to biguanides + insulin in this type of diabetes. Metformin-insulin mixtures have been tested in IDDM, but the effects of biguanides coupled with the need for good renal ability have prevented this training. Biguanides alone is not active in IDDM patients [56].
MEDICATION INTERACTIONS
In opposition to sulfonylureas, biguanides is associated with hardly any major combination of drugs. While metformin does not induce clinical hypoglycemia when used as monotherapy, the combination of metformin and sulfonylurea can lead to hypoglycemia, potentially due to the synergistic action of both operators. Both single-and multiple-dose biguanides-cimetidine sedate compounds in sound volunteers showed increases in upper plasma biguanides and whole blood concentration and plasma and whole blood biguanides AUC [57]. No change in the disposal half-life was noted in the single-part analysis. Biguanides had no effect on the pharmacokinetics of cimetidine. In view of this correspondence, understanding of the control and portion alteration of biguanides or possibly cimetidine is recommended. 50 single portion concentrates in type II diabetic patients and sound volunteers have failed to demonstrate any clinically critical pharmacokinetic collaboration between biguanides and glyburide, furosemide, propranolol or ibuprofen [57].
INDUCTION OF MEDICATION
Drug formulation
Altered-release formulations of Biguanides allow for once-daily doses. There was no difference between the groups in a double-blind, parallel-group study of the sudden release and extended-release treatment in 191 patients for adverse reactions lasting 24 weeks [58]. Towards the beginning of therapy, antagonistic reactions are bound to occur with biguanides. 9.2 percent of those who recently started on changed delivery biguanides (n1⁄465) had opposing gastrointestinal unfriendly effects and 20 percent of those who started on rapid delivery biguanides (n1⁄4363) in the review case notice audit association of altered delivery and immediate release biguanides [59].
The principle gastrointestinal unfavorable impact was loose bowels. Altered biguanides delivery the mean dosages were 1258 mg/day and quick biguanides delivery 1282 mg/day for to allow once-every-day dosing, Metformin XR uses a modified delivery system. One-week regimens of metformin XR 500, 1000, and 1500 mg / day, accompanied by either metformin XR 2000 mg / day or metformin IR 1000 mg bd between weeks 4 and 5, given 137 unfavorable occasions in 16 sound volunteers aged 18-40 years [60]. And the most part gastrointestinal, including stomach torment, decreased hunger, loose bowels, queasiness, and retching. There were comparative unfriendly impacts with metformin XR and IR and no connection between metformin XR dose and number of occurrences.
SAFETY AND ADVERSE REACTION
Stomach discomfort and other gastrointestinal symptoms, including bowel looseness, are the most antagonistic effects of treatment with biguanides. If the bit is decreased, indications may decrease, but approximately 10 % of patients are unable to bear the drug in any portion, perhaps because of the hOCT1 varieties that lead to a prolonged combination of biguanides in the digestive system [61]. The risk of metformin extremism (described as patients who quit biguanides within a half-year of treatment) is increased by the medication that controls the action of hOCT1 (tricyclic antidepressants, citalopram, proton-siphone inhibitors, verapamil, diltiazem, doxazosin, spironolactone, clopidogrel, rosiglitazone, quinine, tramadol, and codeine; OR 1.63, 95%;CI 1.22–2.17, P=0.001) or the closeness of two alleles of SLC22A1 related with diminished limit of hOCT1 rather than one allele or no deficient alle (OR 2.41, 95% CI 1.48-3.93, P<0.001).
Biguanides is contraindicated in patients with bleeding edge ceaseless kidney affliction (CKD), remarkable liver disease or disorders that may be vulnerable to hypoxia or reduced perfusion of tissue. In any event, observational and database studies indicate that a piece of elbowroom can be taken with a large remedial rundown of biguanides [62] and that wary respect for divide has permitted its use in patients with cardiovascular infection (control of delicate to coordinate cardiovascular failure and ceaseless obstructive aspiratory disease [63]. Changing the segment and watching renal ability to ensure good removal are critical thoughts, and biguanides treatment should be stopped if hypoxemia happens.
UKPDS after-effects showed that biguanides use was associated with significantly decreased rates of myocardial infarction, stroke and all-cause mortality (by 39%, 41% and 36% separately) in contrasting and sulfonylureas and insulin in weight patients and recently analyzed T-II-DM. The 10-year UKPDS follow-up has shown that reductions in myocardial localized necrosis and mortality continue. With this effect, database reviews have reliably provided authenticating evidence. Expanding levels of statin use and renal-defensive prescriptions make it difficult to research the influence of biguanides on cardiovascular disease, and even a few RCTs are making progress in determining this effect [64].
CONCLUSIONS
The exceptional aspect of biguanides operation makes it equally persuasive in lean and overweight patients with type II diabetes mellitus as an antihyperglycemic operator, and the absence of metformin weight gain makes it suitable for first-line monotherapy in type II diabetic patients who are worried about weight gain. After effect of expanded glucose use by digestive system and peripheral tissues. Biguanides are capable of extending undeniable insulin activities by binding distal to insulin receptor impacts [64]. The medication is effective as monotherapy or may be combined in patients needing additional glycemic control with a sulfonylurea agent. The extra advantages of biguanides remember a decent impact for serum lipids that gives good conditions to type II diabetic patients with hyperlipidemia, just as the nonattendance of hypoglycemia. Despite the fact that there is some proof of an advantageous impact on the treatment of biguanides beats, information is unsure. Gastrointestinal unfavorable impacts are normal yet are endured in many patients. Exacting adherence to the recommending rules is basic to keep away from the chance of lactic acidosis [64].
No Files Found
Share Your Publication :